Notes For All Chapters Science Class 7
Study Material and Notes of Ch 5 Physical and Chemical Changes Class 7th Science
Topics in the chapter
Types of changes
Physical change
Chemical change
Copper Sulphate Reaction
Rusting
Crystallization
Types of Changes
→ Changes can broadly be classified into two types − physical and chemical.
Physical Change
→ Properties such as shape, size, colour and state of a substance are called its physical properties.
→ A physical change in which a substance undergoes a change in its physical properties is called a physical change.
Chemical Change
→ A change in which one or more new substances are formed is called a chemical change.
→ A chemical change in which a substance undergoes a change in its chemical properties is called a chemical change.
• Burning of Magnesium Ribbon
→ Magnesium ribbon burns with a brilliant white and leaves behind a powdery ash when completely burnt.
Magnesium (Mg) + Oxygen (O2) → Magnesium oxide (MgO)
Dissolving magnesium ash in water
Magnesium oxide (MgO) + Water (H2O) → Magnesium hydroxide [Mg(OH)2]
• Difference between Physical and Chemical Change
| Physical Change | Chemical Change |
| 1. The chemical composition of a substance does not change. | 1. The chemical composition of a substance changes. |
| 2. Most changes are reversible. | 2. Most changes are irreversible. |
| 3. No new substances are formed. | 3. No new substances are formed. |
| For example: Ice → Water → Steam | For example, Paper → Ashes |
→ Burning a candle is a combination of physical and chemical change.
Copper Sulphate Chemical Reaction
→ When copper sulphate reacts with iron, the colour of the solution changes from blue to green due to the formation of iron sulphate.
• Copper Sulphate solution (blue) + Iron → Iron Sulphate solution (green) + Copper (brown deposit)
Rusting
→ Rusting is an example of chemical change.
Iron (Fe) + Oxygen (O2, from the air) + water (H2O) → rust (iron oxide Fe2O3)
→ Presence of both air and water is essential for rusting to take place.
→ Rusting can be prevented by cutting the contact of either air or water or both with iron.
Protection from Rusting
→ The same can be done by greasing, oiling, painting, and galvanizing iron.
→ The process of depositing a layer of zinc on iron is called galvanization.
→ Rusting can also be checked by alloying iron with other elements. Stainless steel is an alloy of iron with carbon, chromium, nickel, and manganese.
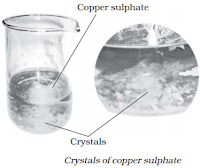
Crystallization
Crystallization is an example of physical change. The process of crystallization is used for
purification of some substances.
NCERT Solutions of Physical and Chemical Changes Class 7



Physical Change Chemical Change
1. The chemical composition of a
substance does not change. 1. The chemical composition of a
substance changes.
2. Most changes are reversible. 2. Most changes are irreversible.
3. No new substances are formed. 3. No new substances are formed.
For example:
Ice → Water → Steam For example,
Paper → Ashes
there is no new substance is formed in chemical change 3rd point
Loved it I am also a topper of the class and I study with this app by recommend you to study with this because in the Convent School like I am studying in Carmel Convent so it works are lot💥❣❣💯💯
Yes, this is a really great app! It has made studying so much easier for me. I had an exam yesterday, and honestly, I hadn’t studied at all. But then I used evidyarthi to get notes, and the notes were so well-made and helpful that I scored 25 out of 25! Thank you so much to the creators of this app!
I Love these notes it’s makes me easily to understand the chapter 5.. tomorrow is my science exam and I searched previous year question papers on chart gpt they shared your link… Thanks for this✌️💫😎☺️☺️
It is such a nice app
I write my all subjects notes with this app
It’s a very good app but please also provide ppt notes of every subject like science,history,
😍😍😍😍😍😍
Evidyarthi is a very good app for study. I write all subjects note from here like history, geography, political science, and science.
I am thanking to evidyarthi for providing us all the notes and question answer.
Thanks a lot for helping us in our study…………..
Evidyarthi is Very good app, too much good