Notes For All Chapters Chemistry Class 12 CBSE
1. The classes of organic compounds containing carbonyl group (CO) as the functional group are aldehydes, ketones, carboxylic acids and their derivates. These are collectively called carbonyl compounds.
2. Nature of carbonyl group: Oxygen atom in carbonyl group is far more electronegative than carbon atom. As a result, the oxygen atom tends to attract the electron cloud of the π-bond towards itself, i. e., the π-electron cloud of >c = O is unsymmetrical.
Hence carbonyl carbon acquires positive charge and carbonyl oxygen carries negative charge. Thus, the carbonyl group is polar in nature.
3. Methods of preparation of Aldehydes and Ketones:
(a) By controlled oxidation of primary and secondary alcohol, aldehydes and ketones are produced.
(b) By dehydrogenation of alcohols : Primary alcohols on dehydrogenation produce aldehydes while secondary alcohols produce ketones.
4. Preparation of Aldehydes:
(a) Acyl chloride (acid chloride) is hydrogenated using, palladium on barium sulphate which is partially poisoned by the addition of S or quinoline. This reaction is called Rosenmund reduction. This method is used to prepare aldehydes.
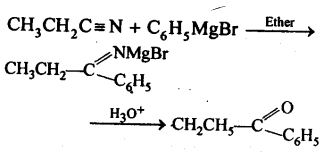
(b) N itriles are reduced to corresponding imine with stannous chloride in the presence of hydrochloric acid, which on hydrolysis give corresponding aldehyde. This reaction is called Stephen’s reduction.
Chromyl chloride (CrO2ClO2) oxidises methyl group of toluene to a chromium complex, which on hydrolysis gives corresponding benzaldehyde. This reaction is called Etard reaction.
(d) When benzene or its derivatives is treated with CO and HCl in the presence of anhydrous AlCl3 or CuCl, it gives benzaldehyde or substituted benzaldehyde. This reaction is called Gatterman-Koch reaction.
5. Preparation of Ketones:
(a) Treatment of acyl chlorides with dialkyl cadmium, prepared by the reaction of cadmium chloride with Grignard reagent, gives ketones.
(b)From nitriles:
When benzene or substituted benzene is treated with acid chloride in the presence of anhydrous A1C13, the corresponding ketone is formed. This reaction is known as Friedel-craft’s acylation reaction.
Properties of aldehyde and ketones
(a) Aldehydes are much more reactive than ketones in nucleophilic addition reactions.
(b) Nucleophilic addition reactions: Aldehydes and ketones undergo nucleophilic addition reactions onto the carbonyl group with a number of nucleophiles such as HCN, NaHSO3, alcohols, ammonia derivatives and Grignard reagents.
(c) Reduction to alcohols: Aldehydes and ketones on reduction gives primary and secondary alcohols respectively.
(d) The carbonyl group of aldehydes and ketones is reduced to CH2 group on treatment with zinc amalgam and concentrated hydrochloric acid (Chemmenson reduction) or with hydrazine followed by heating with NaOH or KOH in high boiling solvent such as ethylene glycol (Wolff-Kishner reduction).
(e) Tollen’s reagent (ammonical silver nitrate) oxidises aldehyde and the silver ions are reduced to silver which appear as a bright silver mirror on the side of the test tube ketones do not give this test.
(f) Aldehydes reduce Fehling’s solution to form a red precipitate of cuprous oxide. Fehling’s solution is obtained by mixing a solution of copper sulphate and a solution of sodium hydroxide and sodium potassium tartrate. Ketones do not reduce Fehling’s solution..Hence no precipitate is formed.
(g) Aldehydes and ketones having atleast one α-hydrogen atom undergoes a condensation reaction when warmed with dilute alkali to form β -hydroxy aldehydes or β-hydroxy ketones respectively. The reaction is known as aldol condensation.
(h) The condensation of a mixture of two different aldehydes or/and ketones each having an a-hydrogen atom, in presence of dilute alkali gives a mixture of four products. The reaction is known as cross aldol condensation.
6. Cannizzaro reaction: Aldehydes which do not have an a-hydrogen atom, undergo self-oxidation and reduction (disproportionation) reaction on treatment with concentrated alkali. In this reaction, one molecule of the aldehyde is reduced to alcohol white another is oxidised to carboxylic acid salt.
7. Electrophilic substitution reaction: It takes place at the ring in which the carbonyl group acts as a deactivating and meta-directing group.
8. Methods of preparation of carboxylic acids:
(a) From oxidation of primary alcohols and aldehydes.
(b) Aromatic carboxylic acids can be obtained by side chain oxidation of alkyl benzenes.
(c) From hydrolysis of nitriles and amides:
(d) From reaction of Grignard reagents with carbon dioxide:
9. Aliphatic carboxylic acids having up to four carbon atoms are miscible in water due to the formation of hydrogen bonds with water.
10. The solubility decreases as the number of carbon atoms increases.
(i) The electron withdrawing group (Cl, NO2, CN, etc.) stabilises the carboxylate anion by dispersing the negative charge of the carboxylate anion, RCOO–, and thus increases the acidic strength.
(ii) The presence of electron donating substituent such as alkyl group intensifies the negative charge on the RCOO– anion and destabilises it thereby making the carboxylic acid less acidic.
(iii) Carboxylic acids having an α-hydrogen are halogenated at the α-position on treatment with chlorine or bromine is the presence of small amount of red phosphorus to give α- chloro or α – bromo carboxylic acids. This reaction is known as Hell-Volhard Zelinsky Reaction.
Leave a Reply