Chemical Reactions and Equations
Solutions For All Chapters Science 10 CBSE
NCERT TEXTBOOK Solutions for Class 10 Science
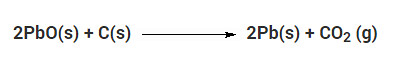
Question 1
Which of the given statements about the reaction below are incorrect?
a. Lead is getting reduced
b. Carbon dioxide is getting oxidised
c. Carbon is getting oxidised
d. Lead oxide is getting reduced
(i) (a) and (b)
(ii) (a) and (c)
(iii) (a), (b) and (c)
(iv) all
Solution:
(i) (a) and (b)
Question 2
The above reaction is an example of a
(i). Combination reaction
(ii). Double displacement reaction
(iii). Decomposition reaction
(iv). Displacement reaction
Solution:
(iv) Displacement reaction
Question 3
What happens when dilute hydrochloric acid is added to iron fillings? Tick the correct answer.
a. Hydrogen gas and iron chloride are produced
b. Chlorine gas and iron hydroxide are produced
c. No reaction takes place
d Iron salt and water are produced
Solution:
Hydrogen gas and iron chloride are produced
Question 4
What is a balanced chemical equation? Why should chemical equations be balanced?
Solution:
An equation for a chemical reaction is an equation in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and the products. In other words, the mass and the charge are balanced on both sides of the reaction.
Question 5
Translate the following statements into chemical equations and then balance them.
a. Hydrogen gas combines with nitrogen to form ammonia
b. Hydrogen sulfide gas burns in air to give water and sulfur dioxide
c. Barium chloride reacts with Aluminium sulfate to give Aluminium chloride and a precipitate of barium sulphate
d. Potassium metal reacts with water to give a potassium hydroxide and hydrogen gas
Solution:
a. 3H2 + N2 → 2NH3
b. 2H2S + 3O2 → 2H2O + 2SO2
c. 3BaCl2 + Al2(SO4)3 → 2AlCl3 + 3BaSO4
d. 2K + 2H2O → 2KOH + H2
Question 6
Balance the following chemical equations.
a. HNO3 + Ca(OH)2 → Ca(NO3)2 + H2O
b. NaOH + H2SO4 → Na2SO4 + H2O
C. NaCl + AgNO3 → AgCl + NaNO3
d. BaCl2 + H2SO4 → BaSO4 + HCl
Solution:
a. 2HNO3 + 2Ca(OH)2 → Ca(NO3)2 + 2H2O
b. 6NaOH + 3H2SO4 → 3Na2SO4 + 6H2O
C. NaCl + AgNO3 → AgCI + NaNO3
d. BaCl2 + H2SO4 → BaSO4 + 2HCl
Question 7
Write the balanced chemical equations for the following reactions:
(a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water
(b) Zinc + Silver nitrate → Zinc nitrate + Silver
(c) Aluminium + Copper chloride → Aluminium chloride + Copper
(d) Barium chloride + Potassium sulphate → Barium sulphate + Potassium chloride
Answer:
(a) Ca (OH)2 + CO2 → CaCO3 + H2O(b) Zn + 2AgNO3 → Zn(NO3)2 + 2 Ag(c) 2Al + 3 CuCl2 → 2AlCl3 + 3 Cu(d) BaCl2 + K2SO4 → BaSO4 + 2KCl
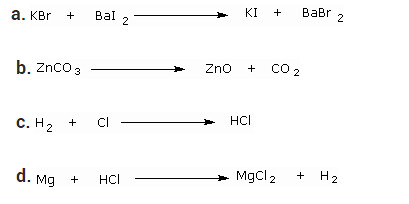
Question 8
Write the balanced chemical equation for the following and identify the type of reaction of each case.
Solution:
a. 2KBr + BaI2 → 2KI + BaBr2 — Double Displacement reaction
b. ZnCO3 → ZnO + CO2 — Decomposition reaction
c. H2 + Cl2 → 2HCl — Combination reaction
d. Mg + 2HCl → MgCl2 + H2 — Displacement reaction
Question 9
What is meant by exothermic and endothermic reactions? Give examples.
Solution:
An exothermic reaction is one that releases heat.
If 1 mole of N2 molecule reacts with 1 mole of O2 molecule, the heat of 184 KJ has to be supplied to initiate the
reaction to give 1 mole of NO molecule. This means that the bonds between N – N and O – O are so strong that they
do not break easily. N2 has triple covalent bond between the two N atoms. O2 has a double covalent bond. Thus
energy has to be put into the reaction to break the strong bonds. Thus the above reaction is a good example of an endothermic reaction.
Question 10
Why is respiration considered an exothermic reaction?
Solution:
During respiration, glucose combines with oxygen in the cells of our body to form carbon dioxide and water along with the production of energy.
Glucose + oxygen → carbon dioxide + water + energy
It is an exothermic reaction, because energy is produced during this process.
Question 11
Why are decomposition reactions called the opposite of combination reactions? Write equations for decomposition reactions.
Solution:
Essentially, decomposition reactions are the opposite of combination reactions. A compound decomposes (i.e., “splits-up”) into two or more compounds and/or elements. For example, mercury (II) oxide will, decompose into mercury metal and oxygen, upon heating:
2HgO → 2Hg + O2
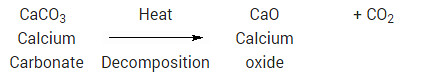
Since heat had to be added to make this reaction occur, it is an endothermic reaction. Most decomposition reactions are endothermic. Another example of decomposition reaction is the heating of calcium carbonate (sea shells, chalk):
Question 12
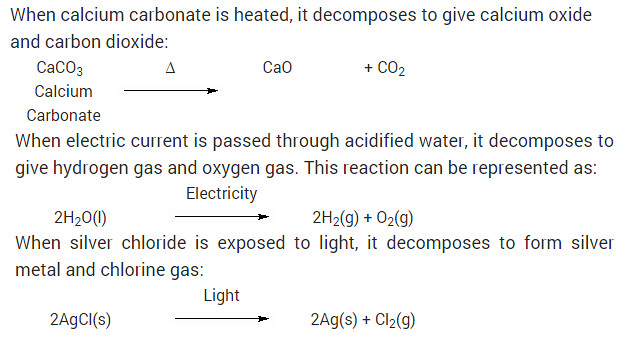
Write one equation each for decomposition reactions in which energy is supplied in the form of heat, light or electricity?
Solution:
When calcium carbonate is heated, it decomposes to give calcium oxide and carbon dioxide:
Question 13
What is the difference between displacement and double displacement reactions? Write relevant equations for the above?
Solution:
The difference between the displacement and double displacement reactions is that in a displacement reaction one element displaces another by virtue of it being more reactive whereas in a double displacement two anions and two cations switch places between two compounds respectively.
For example:
CuSO4 (aq) + Zn(s) → ZnSO4 + Cu(s)
Blue copper sulphate solution reacting with solid zinc will give rise to colourless zinc sulphate solution and solid copper. Thus Zn displaces Cu in the salt form. Zn is more reactive than Cu.
An example of double displacement reaction is the reaction between sodium carbonate and calcium chloride, both in aqueous
Solution:
Na2CO3 (aq) + CaCl2 (aq) → CaCO3 (ppt) + 2NaCl(aq)
Question 14
In the refining of silver, the recovery of silver from silver nitrate solution involves displacement by copper metal. Write down the reaction involved.
Solution:
Question 15
What do you mean by a precipitation reaction? Explain by giving examples:
Solution:
A precipitation reaction is a reaction in which soluble ions in separate solutions are mixed together to form an insoluble compound that settles out of solution as a solid. This insoluble compound is called a precipitate.
An example of a precipitation reaction
Aqueous silver nitrate (AgNO3), when added to a solution containing potassium chloride (KCl), precipitates a white solid, and silver chloride is observed.
AgNO3 (aq) + KCl (aq) → AgCl (s) + KNOO3 (aq)
The silver chloride (AgCl) has formed a solid, which is observed as a precipitate.
Question 16
A shiny brown colored element ‘X’ on heating in the air becomes black in color. Name the element ‘X’ and the black coloured compound formed.
Solution:
The shiny brown colored element X is copper metal (Cu). When copper metal is heated in air, it forms a black colored compound copper oxide. So, the black colored compound is the copper oxide or copper (II) oxide, CuO.
Question 17
Why do we apply paint on iron articles?
Solution:
Rust is a soft and porous substance, which gradually falls from the surface of an iron object, and then the iron below starts rusting. Thus, rusting of iron (or corrosion of iron) is a continuous process which, if not prevented in time, eats up the whole iron object. So, when we apply paint on iron articles it reduces the rusting of iron.
Question 18
Oil and fat containing food items are flushed with nitrogen. Why?
Solution:
Packaging fat and oil containing foods in nitrogen gas can prevent rancidity. When the fat and oil present in food materials gets oxidised (in air), their oxidation products have an unpleasant smell and taste. When it is surrounded by unreactive gas, nitrogen, there is no oxygen (of air) to cause its oxidation and make it rancid.
Question 19
Explain the following terms with one example each.
a. Corrosion
b. Rancidity
Solution:
a. Corrosion is the process in which metals are eaten up gradually by the action of air, moisture or a chemical on their surface. Corrosion is caused mainly by the oxidation of metals by the oxygen of air.
Example: Rusting of iron is the most common form of corrosion. When an iron object is left in damp air for a considerable period of time, it gets covered with a red-brown flaky substance called ‘rust’. This is called rusting of iron.
b. The condition produced by aerial oxidation of fat and oil in food which is marked by an unpleasant smell and taste is called rancidity.
Example: Rancidity can be retarded by keeping food in a refrigerator.
The refrigerator has a low temperature inside it. When the food is kept in a refrigerator, the oxidation of fat and oil in it is slowed down due to low temperature. So, the development of rancidity due to oxidation is retarded.
Question 20
Why should a magnesium ribbon be cleaned before burning in air?
Solution:
To remove the oxide layer and facilitates rapid burning.
Question 21
Write the balanced equation for the following chemical reactions.
i. Hydrogen + Chloride → Hydrogen chloride
ii. Barium chloride + Aluminium sulphate → Barium sulphate + Aluminium chloride
iii. Sodium + water → Sodium hydroxide + Hydrogen
Solution:
i. H2 + Cl2 → 2HCl
ii. 3BaCl2 + Al2(SO4)3 → 3BaSO4 + 2AlCl3
iii. 2Na + 2H2O → 2NaOH + H2
Question 22
Write a balanced chemical equation and state symbols for the following reactions.
a. Solutions of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and the solution of sodium chloride
b. Sodium hydroxide solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium chloride solution and water.
Solution:
Question 23
A solution of a substance ‘X’ is used for white washing.
Name the substance ‘X’ and write its formula.
(i) Write the reaction of the substance ‘X; named in (ii) above with water
Solution:
Question 24
Why does the colour of copper sulphate solution change when an iron nail is dipped into it?
Solution:
In this reaction, iron displaces copper from copper sulphate solution. The deep blue colour of copper sulphate solution fades due to the formation of light green solution of iron sulphate. A red-brown coating of copper metal is formed on the surface of the iron metal. This displacement reaction occurs because iron is more reactive than copper.
Question 25
Identify the substances that are oxidised and the substances that are reduced in the following reactions.
i. 4Na(s) + O2 (g) → 2Na2O(s)
ii. CuO(s) + H2(g) → Cu(s) + H2O(l)
Solution:
i. 4Na(s) + O2 (g) → 2Na2O(s)
Here oxygen is added to sodium. The addition of oxygen is Called oxidation. So the substance that is oxidized is sodium Na.
ii. CuO(s) + H2(g) → Cu(s) + H2O(l)
In this reaction, copper oxide (CuO) gives the oxygen required for the oxidation of hydrogen; therefore, copper oxide is the oxidizing agent. Hydrogen is responsible for removing oxygen from copper oxide; therefore, hydrogen is the reducing agent here.
NCERT Intext Questions for Class 10 Science Page Number: 6
Question 1
Why should a magnesium ribbon be cleaned before burning in air ?
Answer:
Magnesium gets covered with a layer of magnesium oxide when kept in air for a long time. This layer hinders the burning of magnesium. Hence, it is to be cleaned before burning.
Question 2
Write the balanced equation for the following chemical reactions.
(i) Hydrogen + Chlorine → Hydrogen chloride
(ii) Barium chloride + Aluminium sulphate → Barium sulphate + Aluminium chloride
(iii) Sodium + Water → Sodium hydroxide + Hydrogen
Answer:
(i) H2 + Cl2 → 2HCl
(ii) 3 BaCl2 + Al2(SO4)3 → BaSO4 + 2 AlCl3
(iii) 2Na + 2H2O → 2NaOH + H2↑
Question 3
Write a balanced chemical equation with state symbols for the following reactions :
(i) Solutions of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and the solution of sodium chloride.
(ii) Sodium hydroxide solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium chloride solution and water.
Answer:
(i) BaCl2 (aq) + Na2SO4 (aq) → BaSO4(s) + 2NaCl (aq)
(ii) NaOH (aq) + HCl(aq) → NaCl(aq) + H2O(l)
NCERT Intext Questions for Class 10 Science Page Number: 10
Question 1
A solution of a substance ‘X’ is used for white washing.
(i) Name the substance ‘X’ and write its formula.
(ii) Write the reaction of the substance ‘X’ named in (i) above with water.
Answer:
(i) The substance whose solution in water is used for white washing is calcium oxide (or quick lime). Its formula is CaO.
Question 2
Why is the amount of gas collected in one of the test tubes in text book Activity 1.7 (i.e., electrolysis of water) double of the amount collected in the other? Name this gas.
Answer:
In Activity 1.7, water is electrolysed to give H2 gas at one electrode and O2 gas at the other electrode.
2H2O(l) → 2H2(g) + O2(g)
Thus two molecules of water on electrolysis give two molecules of hydrogen gas and one molecule of oxygen gas or in other words the amount of hydrogen gas collected would be double than that of oxygen gas.
NCERT Intext Questions for Class 10 Science Page Number: 13
Question 1
Why does the colour of copper sulphate solution change when an iron nail is dipped in it ?
Answer:
When an iron nail is dipped in copper sulphate solution, the displacement reaction takes place. The colour of copper sulphate solution fades due to the formation of light green solution of iron sulphate.
Question 2
Give an example of a double displacement reaction other than the one given in Activity 1.10 (NCERT Text Book).
Answer:
Sodium hydroxide and hydrochloric acid react to form sodium chloride and water.
Question 3
Identify the substances that are oxidised and the substances which are reduced in the following reactions.
(i) 4Na(s) + O2(g) → 2Na2O(s)
(ii) CuO (s) + H2(g) → Cu (s) + H2O(l)
Answer:
(i) Substances oxidised is Na as it gains oxygen and oxygen is reduced.
(ii) Substances reduced is Cu as hydrogen is oxidised as it gains oxygen.
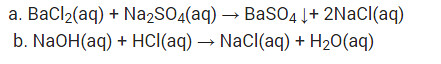

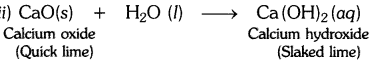
बहुत अच्छा study material है, आपकी website पर 🙏☺️
English medium के लिए भी question answer का PDF बना दीजिए 🙏☺️