Question Answers For All Chapters – General Science Class 8
Exercise
1. Complete the table:
| Property of Metal | Use in Everyday Life |
|---|---|
| Ductility | Used to make wires (e.g., copper, aluminum wires). |
| Malleability | Used to make thin sheets (e.g., aluminum foil, gold sheets). |
| Conduction of Heat | Used in cooking utensils (e.g., copper and aluminum pans). |
| Conduction of Electricity | Used in electrical wiring (e.g., copper, aluminum wires). |
| Sonority | Used in making bells and musical instruments. |
2. Identify the odd term
(a) Gold, silver, iron, diamond → Diamond (It is a nonmetal, while others are metals).
(b) Ductility, brittleness, sonority, malleability → Brittleness (It is a property of nonmetals, while others are properties of metals).
(c) Carbon, bromine, sulfur, phosphorus → Bromine (It is a liquid, while others are solids at room temperature).
(d) Brass, bronze, iron, steel → Iron (It is a pure metal, while others are alloys).
3. Write scientific reasons:
(a) The stainless steel vessels in kitchens have copper coating on the bottom.
- Copper is a good conductor of heat, so it helps in even cooking.
- It also prevents burning of food by distributing heat properly.
(b) Copper and brass vessels are cleaned with lemon.
- Copper and brass develop a greenish layer due to oxidation.
- Lemon contains acid, which removes this layer and makes the metal shine.
(c) Sodium metal is kept in kerosene.
- Sodium is highly reactive and catches fire when exposed to air or water.
- Storing it in kerosene prevents it from coming in contact with air or moisture.
4. Answer the following:
(a) What is done to prevent corrosion of metals?
- Metals are protected by painting, greasing, galvanization (zinc coating), and alloying.
(b) What are the metals that make the alloys brass and bronze?
- Brass = Copper + Zinc
- Bronze = Copper + Tin
(c) What are the adverse effects of corrosion?
- Corrosion weakens metals, reduces their lifespan, and damages buildings, bridges, and vehicles.
(d) What are the uses of noble metals?
- Gold, silver, and platinum are used in jewelry, medicines, electronic devices, and medals.
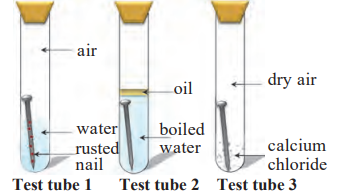
5. Three experiments to study rusting of iron nails:
| Test Tube | Observations |
|---|---|
| Test Tube 1 (air + water) | The nail rusts highly because both air (oxygen) and water are present. |
| Test Tube 2 (boiled water + oil layer, no air) | The nail does not rust because oxygen is removed, and oil prevents air from entering. |
| Test Tube 3 (dry air + calcium chloride, no water) | The nail does not rust because moisture is removed. |
(a) Why is the nail in test tube 2 not rusted?
- No oxygen is present because the water was boiled, and oil prevents air from entering.
(b) Why is the nail in test tube 1 rusted highly?
- Both oxygen from the air and water are present, which cause fast rusting.
(c) Would the nail in test tube 3 get rusted?
- No, because there is no moisture (water), which is necessary for rusting.

Leave a Reply